Comprehensive Analysis of Wool
Composition, Properties, and Unique Characteristics
Chemical structure of Wool
The wool fiber can be simply described as a polypeptide which is produced through condensation of carboxyl and amino groups of 18 different α amino acid residue as monomer having the general formula as given below:

These amino acids are linked to each other by means of peptide bond (-CO-NH-) to form the peptide chain.
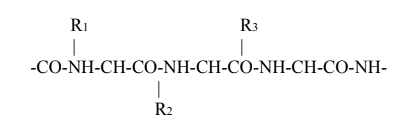
The side groups of this chain may vary to a large extent in size and chemical nature. For instance, some of them may be hydrophobic, some hydrophilic, some acidic or basic.
Physical and Chemical properties of Wool
Physical properties:
Wool fiber varies remarkably in length, crimp, luster, strength and dye uptake depending upon which part of the sheep’s body they came from and the conditions that existed during growth.
Length and fineness:
Merino wool fibers are very fine (17-25 μm) but not very long (60-100), whereas a Lincoln wool is coarser (around 40 μm) diameter but much longer (175-250 mm). The measurement of actual length of a wool fiber is complicated by its crimp. The stretch length of a fiber may be nearly twice that of its natural length. Wool fibers are roughly oval in cross-section.
Crimp:
Wool fibers are unique among natural fibers in having a wavy structure which has great
importance in practical use. It enables the fibers to hold together when twisted into a yarn. This crimp of wool fibers is most pronounced in the fine wool fibers. The best merino wools, for example, will have 30 waves per inch. The elasticity of wool fiber is due to its crimp. Due to its crimp, wool yarns trap air and when used in garments, providing an insulating barrier to loss of body heat. Depending upon the texture and thickness of the fabric, as much as 60 to 80% of the volume of a wool cloth may be air. That’s why wool fabric feels warm.

Luster:
Wool fibers have natural luster which varies in its characteristics, depending on the type of wool. Luster depends very largely on the nature of the fiber surface. The Lincolns, Leicester and other English breeds’ wool have a silky luster. Merino wool does not reflect light so perfectly. They have a delicate luster.

Tenacity:
Wool has a tenacity of 8.8-15 cN/tex in wet state.
Elongation:
Wool has an elongation at break of 25-35% under standard condition and of 25-50% when wet.
Elastic property:
Wool fibers are highly elastic and resilient. Wool fabrics therefore do not crease easily, have good crease recovery and wool garments fit well. The elastic recovery of wool fibers is 65% for 20% extension and almost 100% for short extensions. The natural crimp of the fibers contributes to the elasticity of wool as the fibers return to their wavy form after deformation.
Specific gravity:
Wool is a light-weight fiber of specific gravity 1.32.
Effect of moisture and water:
Wool fiber is hygroscopic and the most hydrophilic of textile fiber. Under ordinary atmospheric conditions, wool will hold 16-18% of its weight of moisture. Under suitable circumstances, wool will absorb about one third of its weight of water. Despite the high regain, wool does not feel damp. Wool can absorb moisture but repels water. The wavy outer layer of wool repels water because of its lower surface energy in comparison to that of water. The pores are so small that the water droplets cannot pass through the fiber surface, but evaporated or molecular water (sweat) can pass through the surface pores. If wool fibers are immersed in water, the fibers swell mainly along their diameter and increase in size according to the degree of wetting. They increase in length by approximately 1.2% and in thickness by approximately 18%.
Wool fibers are associated of millions of keratin molecules, that are aligned one beside the other, held together by chemical and electrical forces. The penetration of water molecules inside the fiber loosen the mutual grip of the molecules lying close alongside each other. The molecules are able to move more easily relative to one another, causing swelling and reducing the tensile strength of the fiber. The wet wool becomes softer and more plastic. The absorption of water is based on the presence of numerous hydrophilic groups.
Cold water does not attack wool fiber chemically, but hot water has considerable effect on wool fiber. When wool fiber is boiled for 2 hours in distilled water, it losses its weight by negligible amount. But boiling for 12 hours decreases 29% of the fiber weight. At temperature, up to 80°C of water, the mechanical properties of fibers do not change considerably, but the rise in temperature between 80-100 °C causes remarkable change in their mechanical behavior. On further increase in temperature, wool fiber dissolves.
Effect of heat:
Dry heat causes less damage to wool than wet heat does. If wool exposed to dry heat at 100-150°C for a long period it dries up and consequently becomes the fiber hard and stiff. It also causes loss of its strength to some degree. But it has been observed that wool fiber retains most of its physical and chemical properties in dry state at below 150°C when returning in moist air. Serious modifications only occur when the temperature exceeds 150°C and the fiber is not in neutral condition.
On the other hand, heating of wool in presence of moisture causes much more pronounced deterioration of wool. Due to moisture, the amount of NH3 and H2S increases rapidly when the temperature goes above 100°C. These two products may cause decomposition of wool fiber.
Contractions of wool fibers produced by heating in sealed tubes with water for 3 hours at
different temperatures are given below-
Temperature | Contraction (%) |
100 | 0 |
115 | 3.5 |
120 | 22 |
130 | 33 |
140 | 38 |
160 | 53 |
Action of sunlight:
The keratin of wool decomposes under the action of sunlight. Exposure to sunlight may cause yellowing or bleach of wool fiber, depending on the relative intensities of radiation at different wavelengths of light. The light source of short wave length (λ=200 to 300nm), being particularly harmful, cause a gradual degradation of the scaly layer and of the fiber. Thus, wool fiber loses its strength and develops a harsh feel.
The fibers exposed to sunlight contain reducing groups (i.e. aldehyde, thiol, etc.) that impair the uniform dyeability of the fibers.
Wool subjected to strong sunlight is particularly sensitive to moisture, alkalis-including soapy water.
Chemical properties
Action of acids:
Hot-diluted or cold-concentrated acid hardly influences the strength characteristics of woolen articles. Concentration acid solutions cause noticeable destruction of wool depending on the duration and temperature of treatment. In identical conditions, organic acids affect wool less than mineral acids.
Almost all acids cause contraction of wool. Contractions of wool fiber after treatment with acids at 100°C are given below:
Treatment | Time (hrs) | Contraction (%) |
3% HCL | 1 | 13.5 |
10% H2SO4 | 0.5 | 13.6 |
Conc. Acetic acid | 2.0 | 9.6 |
Conc. Formic acid | 2.0 | 20.8 |
Wool fiber is able to take up acids and alkalis under simultaneous swelling. The acid swelling, particularly with sulfuric acid, is of technical importance in dyeing the wool fiber. It makes the wool fiber accessible for the large molecules of the dyestuffs.
Vegetable impurities of wool are removed by carbonizing, where wool is treated with about 6% sulfuric acid. It is then baked for 3 min at 150°C. As a result, vegetable impurities become brittle, which are then removed from the fabric.
Effect of alkalis:
The chemical nature of wool keratin as such that it is particularly sensitive to alkaline substances. Wool will dissolve in caustic soda solutions that would have little effect on cotton. In fact, a 3% solution of sodium hydroxide will completely dissolve wool at boil. So that, the scouring and processing of wool is carried out under out under conditions of low alkalinity. Even weakly alkaline substances such as soap or soda are used with care. Alkali also causes the yellowing of wool fibers. Ammonium carbonate, borax, and sodium phosphate are mild alkalis that have minimum effect on wool.
Effect of oxidizing agent:
Oxidizing agents cause considerable change in composition and properties of wool attacking preferentially in crystalline linkage. The break-down of crystalline linkage due to oxidation is highly undesirable as it results in a lower strength, weight loss and reduction of sulfur content of wool fiber. For bleaching purpose, hydrogen peroxide is commonly used as oxidizing agent.
The extent of damage of wool depends on mainly-
• Temperature
• Concentration of H2O2
• Time of treatment
• PH of the bleaching solution
In addition, the presence of small amount of metal such as copper and iron causes the damage of the fiber to an increased extent.
Partial oxidation of wool is affected by halogens. The treatment of wool with halogen is of great interest because,
• it reduces shrinkage in felting
• it increases the capacity of wool for absorbing dyes
• fiber become lustrous
But it may cause the harsh feel of the fiber and yellowing effect. Chlorine is commonly used in anti-shrink process of wool.
Effect of Reducing agents:
Wool is most strongly attacked by reducing agents in an alkaline solution. They attack preferably on crystalline linkage. The extent of reaction of disulfide, groups of wool with reducing agents depends on the pH range 2-6 and increases considerably for further increase in pH.The reduction of crystalline linkages has significant impact on the mechanical behavior of wool fiber. The reduction treatment results in the poor degree of orientation of polymer, but the average crystallite size is increased. When the treatment of wool with bisulfite is carried out for 30 minutes at boil and followed by washing and drying without tension, the lengthof fiber is reduced by 30%, which is mainly due to breakage of disulfide groups of cystine as well as to the salt linkages of the fiber.
Special Properties of Wool
Acid and basic nature:
The specific characteristic of wool is its amphoteric behavior (the ability to appear as a function of the pH value as an acid or as a base). The reason for this amphoteric nature lies in its composition that it contains many cation and anionic groups at the same time.
The basic and acid properties of proteins depend on the amino groups and carboxylic groups found at the ends of the polypeptide chains. In acidic solutions, the carboxylate ions combine with protons to form neutral carboxylic acid groups and the ammonium ion groups make the fiber cation. Conversely, in alkaline solution, reaction with hydroxide ions convert ammonium ion to amino groups and the fiber becomes anionic.
Usually the acid and basic groups of proteins do not balance each other due to the difference in their number and degree of ionization. Acid characteristics prevail over the basic nature to some extent. But at certain pH can exist equal large quantity of cations and anions, so that the electrical charge of the solution is equal to zero, this point is called isoelectric point (ISP). The isoelectric point of wool lies at pH range 4.7-4.9. Wool is very stable at this point and consequently exhibits lowest reactivity at this point.
Felting properties:
The tendency of wool to felt is a distinctive property that is not found in many other textile fibers. The scaly layer of wool fiber is responsible for this property. Washing of woolen articles causes irreversible shrinkage and felting. Mechanical compression and relaxation of the fibers in a woolen fabric during washing allow the edges of wool fibers to migrate only in the direction of the root end. The migrated fibers, owing to its scale structure are interlocked with each other preventing the fiber from returning to its original position. This irreversible shrinkage is called felting. It closes up the fabric structure, making it much more compact and of increased rigidity.
There is no individual theory regarding the reasons of wool felting. In general, it is believed that a combination of a number of factors cause wool to felt. Apart from the theory of the interlocking of the epidermal scales on the surface of the wool, the natural twist of the fiber also contributes to the felting property, which increase the amount of inter-fiber contact. When placed in water or in a saturated atmosphere the fibers get active, tends to twist and revolve quite rapidly until they come to rest. Shrinking and felting are obviously undesirable in a finished article that is going to be repeatedly washed.

Crimp:
Each wool fiber contains hundreds of tiny waves, called crimps or curls, which look like coiled spring and create pockets and gives the wool insulating property and a spongy feel. The springy nature of wool allows it to be stretched up to 50% when wet, 30% when dry and still bounce back to its original shape.
The crimp derives from the difference in tension between the Orth cortex and paracortex of the main tissue. Equality of crimp varies with the type of wool, nature of growth and associated with uniformity, which therefore indicates the quality of wool. The number of crimps in wool also expresses its fineness. In general, the more crimps per inch, the finer the fiber. As for example, the number of crimps per inch in very fine grades wool is 22-30, whereas in fine wool it is 14-22.